
Amino Labs PCR-IT KIT Classroom
Let’s get started
This user guide was created to help you get the most out of your Amino Labs experience. Even if you are familiar with genetic engineering, science, or other Amino Labs™ products, please take the necessary time to read through this guide. This will ensure you practice safe science as well as store, use, and get the most out of your kit. It will also inform you on what to do in the event of a spill or accident.
In the first section, you will learn about the components of your kit, how to store them before and during your experiment, as well as a few tips on activities to complete before you get started. The second section is procedural — these are the step-by-step instructions on how to run your experiment. Make sure to follow for the best success!
The third section covers “what’s next”: how to keep, store, or dispose of any leftover ingredients and general clean-up instructions. The final section is there to help you: a glossary, troubleshooting, and our contact information. Amino Labs is excited to welcome you to the world of genetic engineering and DNA technology with our Zero to Genetic Engineering Hero™ journey, the PCR-it Kit™, and all our easy-to-use, easy-to-succeed-at products!
Following this guide will help ensure that you are getting the most out of your current and future experiences to keep on making new creations with DNA. Have fun!
Practicing safe science
Genetic engineering and life sciences are safe activities when you follow simple guidelines. Read on to ensure you adopt safe practices.
The kit in your hands contains only non-pathogenic ingredients. These are part of the biosafety Risk Group 1 (RG1) (Biosafety Level 1). This is the most benign level and therefore the safest: with these kits, no special containment or training is required in North America. But you must follow these safety guidelines for your safety and the success of your experiment(s)!
We recommend the system and kits for ages 12+, under adult supervision, and 14+ with or without supervision. We recommend that an adult empties the discard container. The cleaning instructions must be strictly followed for safety and experiment success. Make sure to store the kit per the instructions found in this booklet.
- Do not eat or drink near your experiments. Keep your experiment at least 10 feet from food, drinks, etc. Under no circumstances should you eat any of the contents.
- Immunocompromised persons: While the ingredients in these kits are non-pathogenic, some persons, such as immunocompromised persons, can be affected by large numbers of bacteria and should talk to their doctor before doing any experiment.
- Wash your hands before and after manipulating your experiment or the hardware.
- Wear gloves, even when cleaning your station or handling the kit contents (petri plates, loops, etc). This will protect you from your experiment, and your experiment from you. Any latex, nitrile, or general-purpose gloves you can find at the pharmacy will do. After you put your gloves on, be aware of what you touch. Try not to touch your face or scratch itches with your gloved hands!
- If using the DNA PlaygroundTM, PCR machine, and Gel electrophoresis system, place them on a stable work surface. Keep it level at all times.
- Clean up your station, spills, and work surface before and after use. Use a 10% solution of chlorinated bleach, generously sprayed onto a paper towel, and rub onto any contaminated surfaces. (Careful! This can discolor your clothes. A chlorinated spray cleaner also works.
- Find a container to hold the inactivation bag where you will discard used items. An old 1L yogurt container, a large plastic cup, or the like will do. Used items (in science, these are often called consumables) will be loops, tubes, or used petri dishes.
- Eyewear is not provided, but can be worn.
You can download a biosafety poster for your space from www.amino.bio/biosafetyinaction and complete a short safety quiz at www.amino.bio/biosafety-quiz
If you would like to do a short Online lab safety course for your edification, we recommend a Government of Canada course.
Discover the PCR-it KitTM
The PCR-it Kit™ has everything you need for 8 groups of students to undertake two PCR reactions each. These PCR reactions will each create two different DNA fragment sizes. Using the Agarose Gel Electrophoresis Kit or your agarose gel electrophoresis supplies, your students will then run their samples in an agarose gel and analyze their results. You students have now learned to do PCR!
The PCR-it kit is complementary with some of our other learning modules to bring a full, real-world science experiment to your classroom:
- Amino Labs’ PCR-it Kit: Bioinformatics – Use software to calculate the theoretical sizes and sequences of the DNA fragments that are amplified in the PCR-it Kit (Free)
- Amino Labs’ Designing Primers: Advanced Bioinformatics – Use software to create primers to amplify a specific region of DNA from a DNA template (Free)
- Amino Labs’ Designing Primers for Cloning: Advanced Bioinformatics – Use software to design custom primers to amplify DNA for ligation with other DNA (Free)
- Biomanufacturing & SOPs: Standard Operating Procedures, Quality Assurance, and Quality Control – Students undertake a role-playing activity where they manufacture DNA fragments to specifications and read/write SOPs to maintain quality control of their manufactured DNA.
Kit components
Shared materials
- 1 tube of pBR322 template DNA (20 μL): pBR322 is one of the most commonly used E.coli cloning vectors

- 1 tube of pUC19 template DNA (20 μL): pUC19 vector is a small, high-copy-number, E. coli plasmid.

- 1 tube of Premixed pBR322 FWD/REV primers (25 μL): Primers are short pieces of single-stranded DNA that are complementary to the target sequence, in this case, pBR322. This premix contains both the forward and the reverse primer needed for your pBR322 PCR reaction.

- 1 tube of Premixed pUC19 FWD/REV primers (25 μL): Primers are short pieces of single-stranded DNA that are complementary to the target sequence, in this case, pUC19. This premix contains both the forward and the reverse primer needed for your pUC19 PCR reaction.

- 1 tube of Ultra water (300 μL): Ultrapure water (UPW) is water that has been purified to high levels of specification. As a standard, the water contains only H20, as well as a balanced number of H+ and OH- ions. To be classified as ultrapure, water must not contain any detectable endotoxins.

- 1 tube of 6x gel loading dye (40 μL): A gel loading dye is a colored dye that you mix with a DNA sample before running your sample in gel electrophoresis. Loading dyes are useful since they will make it easier to analyze your gel results, as they add color to the sample and they add density to your DNA sample, helping to prevent the DNA from being diffused into the buffer.

For each student group
- A bag of “2x Mastermix” PCR tubes: A premixed, ready-to-use solution suitable for many PCR amplification experiments. It contains everything needed for a standard PCR reaction: Taq DNA Polymerase, dNTPs, Mg2+, and Reaction Buffer. Only primers and template DNA need to be added! Each student group gets 2 tubes.

- A bag of “Agarose gel loading” empty PCR tubes: Small, sterile tubes with snap-shut caps made specifically for use in polymerase chain reaction (PCR) experiments. Each student group gets 2 tubes

Unpacking and storing kits
For a better shelf life and successful experiments, place your PCR-it Kit™ in a standard refrigerator at around 4°C.
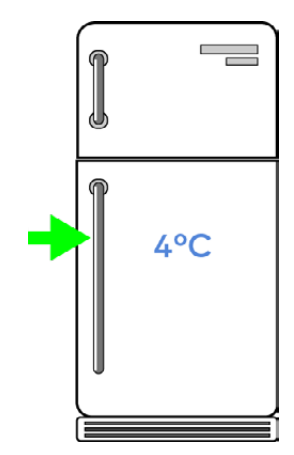
If your refrigerator is not science-only, we recommend placing your science experiments inside a sealed plastic container before placing them in the refrigerator, especially once your kit is open.
Do Not Freeze your kit!
Necessary equipment
Equipment/Supplies required (not included in this kit):
- Thermocycler/PCR machine: 1 per class
- DNA gel electrophoresis tank: 1 per class or 1 per lab group
- Power supply for electrophoresis: 1 per class or 1 per lab group
- Agarose gel casts and combs: 1 set per lab group
- 1 Amino Labs Agarose Gel Electrophoresis Kit or 1 agarose gel per lab group and matching buffer for your gel tank.
- Micropipette (P10): 1 box per lab group
- Pipette tips (P10): 1 box per lab group is ideal, but 1 box/class has enough tips for the experiment.
Necessary safety supplies
- Disposable container 500ml-1L
to hold tubes and other contaminated waste: 1 per station - Latex or nitrile gloves,
like the ones found at a pharmacy. 1 pair/student if students. - Chlorinated bleach spray
1 to share in the classroom (or you can mix a 10% solution: 1 part bleach to 9 parts water in a spray bottle) - Bleach ~500 mL
to inactivate all the experimental materials at the end of the experiment.
Timeline
This PCR Kit experiment is made up of 3 hands-on activities that can take place over 1 half day or 2 class periods. The experiment can also go further with an optional 3 periods for bioinformatics activities.
- Getting your PCR Samples ready
Day 1, 20 minutes - Running your PCR samples in a PCR machine
Day 1, 90 minutes*
*If you are doing the bioinformatics activity, completing the first one while the PCR reaction is running works well.
Bioinformatics activity 1: Calculating your DNA Fragment size and sequences: Use software to calculate the theoretical sizes and sequences of the DNA fragments that are amplified in the PCR reaction. Complete this optional activity before running the gel. - Analysing results with gel electrophoresis
Day 2, 60 minutes
Bioinformatics activity 2: Analyzing the DNA bands on your gel . Generally, measuring DNA requires expensive equipment that classrooms don’t have. Use software to get a good estimation of the amount of DNA in an agarose gel.. Advanced activity to be completed after running your gels.
Bioinformatics Activity 3: Designing Primers:
Use software to learn how to create primers to amplify a specific region from a DNA template. An advanced activity that can be completed at any time
Recommended pre-labs
Amino Labs has many resources that should be used by your students before they complete the hands-on experiment to maximize their understanding and success. These pre-labs are meant to ensure your students know, understand, and complete all the experiment steps. Completing the pre-labs also minimizes the number of questions your students will have during the hands-on experiment.
- Pipette-it Kit: Learn to pipette like a pro
Get the Kit or use your supplies to follow the activity instructions
If your students are new to pipetting, this easy exercise can be done with or without the Amino Labs Pipette-it Kit. To complete the practice exercise, you will need pipettes, pipette tips, empty 0.5 and 1.5 mL tubes, water, and a scale. - Electrophoresis-it Kit: Learn gel electrophoresis
Explore the gel electrophoresis experiment kits..
If your students are new to gel electrophoresis, we have several fun experiments that can guide them through learning to cast gels, running them, using ladders, and analyzing their results. These experiments included background theory and quizzes to help make sure your students are gel electrophoresis pros!
Teacher Experiment Setup
Prepare your classroom space.

Make sure the class has access to a microwave before starting, as they will need this to make their gels if they are using the Amino Labs agarose gel electrophoresis supplies.
- Have students download/print the manual and read through Practicing safe science, the experiment protocol, and the Glossary pages.
- Set your PCR machine up near the students’ work stations. Make sure the equipment is level and on a stable surface. Refer to the instruction manual to make sure you know how to use your equipment safely.
- Set one permanent marker, one discard container er, and one P10 pipette with a tip box per work station on clean work surfaces. You can use chlorinated bleach spray, wipes, or a 10% bleach solution.
Note: If you do not have enough pipettes for all the student groups to have access to their own, set them up in a common area with the shared materials. - Distribute 2 tubes of Mastermix and 2 empty tubes to each student/student team. Keep the shared materials in a common area so all students can access them when needed.
- Ask the student to use the discard container to dispose of:
- any used pipette tips,
- all empty tubes,
Paper, plastic packaging, and gloves should be dispodisposedn the regular garbage or recycling bin. After each day’s experiment or at the end of the entire experiment, have students pour the contents of their discard container into an inactivation bag (in the shared materials bag). Follow the instructions at the end of the manual to inactivate.
- Ask the students to put on their gloves and wipe down their work surface with chlorinated bleach spray, wipe, or 10% bleach solution if needed.
- After the students complete the experiment, follow the Storage, discard & clean up procedures with them.
If you are saving the tubes of DNA, primers, loading dyes, ultrawater, and/or mastermix for a future experiment, place them back in their ziploc bag after use and refrigerate. We recommend you use a sealed plastic container to store all your experiment materials inside a refrigerator if you also use this refrigerator to store food or drinks. If you are not saving them, place the open tubes in a discard container and dispose of them after all the student teams have used them.
Student’s Experiment Protocol
Prepare your PCR samples
Day 1, 25 minutes

Prepare
- Locate your two empty PCR tubes and two 2x Mastermix PCR tubes, or collect them from your teacher.

- Use the permanent marker to label the two Mastermix tubes: one with pBR322 and one with pUC19. Also include your initials or team symbol so you can identify your tubes later on.

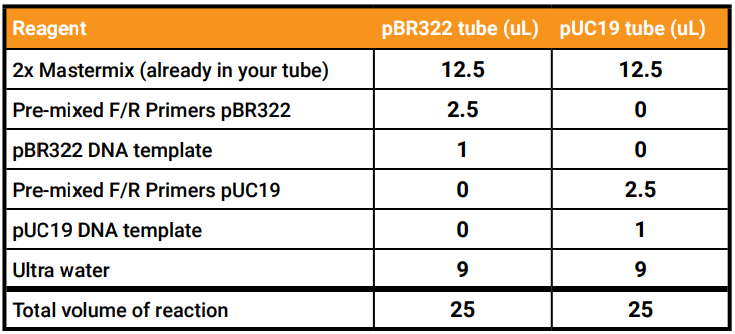
Add your reagents - Using proper pipetting etiquette, add all the PCR ingredients to your tubes to ready them for a PCR reaction according to the table below.
 Mix your sample
Mix your sample - After adding all the components together, mix well by either pipetting up and down or by closing the tube and flicking it around, followed by centrifuging for 5-10 seconds.
Run your PCR samples in a PCR machine
Day 1, 50 minutes

Prepare
- Make sure you have labelled your tubes so you can identify yours from other student groups

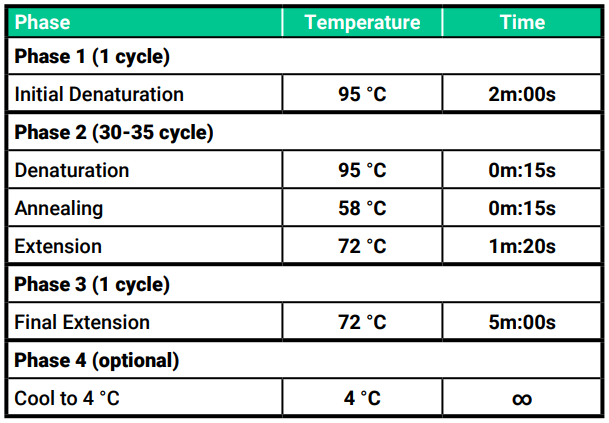
- Place your tubes into a thermocycler (PCR machine) and program your PCR machine as follows:
 Run your PCR samples.
Run your PCR samples. - Run your reaction. The time to complete the reaction will be about 90 minutes depending but it could be higher depending on your PCR machine.
Store your PCR reactions. - Congratulations! You’ve just completed a PCR reaction and hopefully amplified some DNA fragments. The samples in your tubes are now called PCR products. You can store your PCR products in the freezer until you are ready to continue the experiment. If you have time (~1 hour), you can continue right away. Otherwise, you can keep your PCR products in the freezer for up to 1 year before completing the next step.
Bioinformatics Activity 1: Calculating DNA fragment size and sequences
30-60 minutes

Prepare
- Install the ApE software on your computer by going to: https://jorgensen.biology.utah.edu/wayned/ape/
If you need, you can watch how to download and install the software using our quick YouTube video. - Download the exercise file at amino.bio/pcr-it-bioinformatics-files
Watch the video & complete the exercise - Go to this YouTube link, Amino Labs’ Bioinformatics: Calculating DNA fragment size and sequences with A,,, and watch the video to follow along and complete the exercise. Click on the link to go to the YouTube page.
Analyze your PCR products in a gel
Day 1 or 2, 60 minutes

Prepare
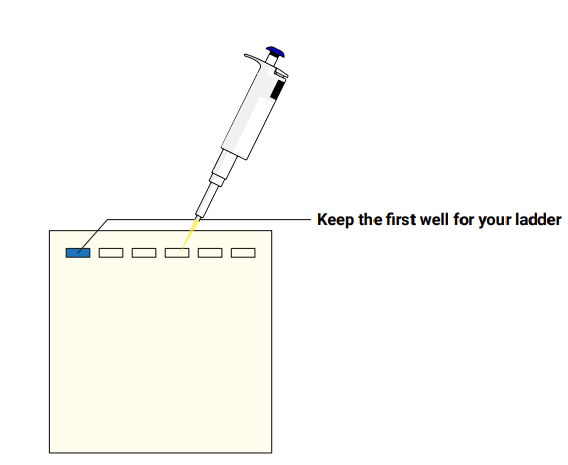
- Follow the instructions for the Amino Labs Agarose gel kit or from your other agarose gel suppliers to cast agarose gels with an 8-tooth comb. You will be using two wells for your samples and one well per gel to include a 1kb ladder or similar. Up to three student can run their samples in the same gel.


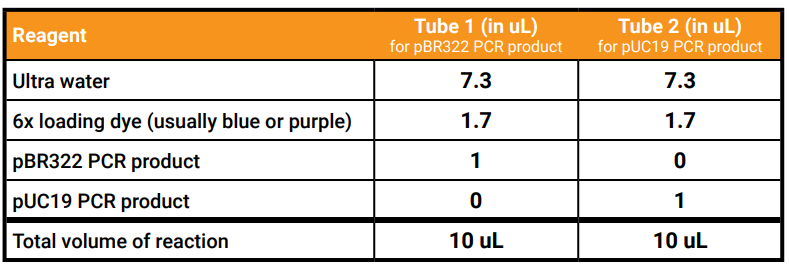
- Use the permanent marker to label the two empty tubes: 1, which you will use for pBR322 PCR product, and 2 for pUC19 PCR product.
- Use the following recipe to prepare your PCR products for the agarose gel:
 Load your PCR products and ladder into a gel..
Load your PCR products and ladder into a gel.. - Still following the instructions from your gel kit, add 10 μL from each of your prepared samples from tubes 1 and 2 to a well in an agarose gel. You can share the gel with other teams, but keep the first well of the gel free for your ladder. Remember to note in your lab book which sample is loaded where!
- Add a 1Kb ladder (or similar) to the first well of the gel. Only one ladder is needed per gel!
Run your gel - Run your gel according to the instructions in your agarose gel kit.
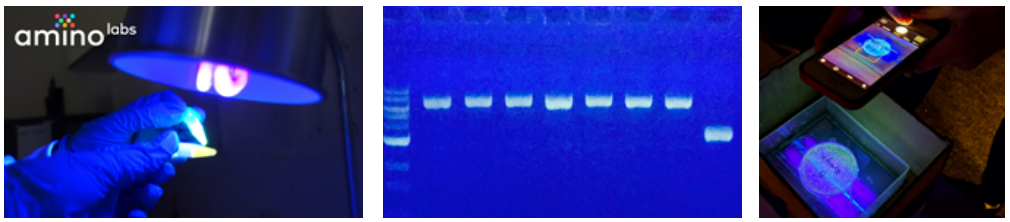
Visualize your gel - Using your transiluminator, visualize your gel to see if the PCR reaction worked as expected. We recommend taking a photo of your gel for further analysis. Make sure not to use any photo filters when taking the photo.
Bioinformatics Activity 2: Analyzing DNA bands in a gel using software

Prepare
- Install the Fiji software on your computer by going to https://fiji.sc/ or use the browser version of the software at https://ij.imjoy.io/
If you need, you can watch how to download and install the software using their tutorial video.
Watch the video & complete the exercise.e - Go to this YouTube link: Amino Labs Bioinformatics: Analyzing DNA Bands with FIJI and watch the video to follow along to complete the exercise.
If you are using the web browser version of ImageJ, note that @ 2:35 in the video, you’ll need to click file in the upper left corner, and then open and select a local file. This will allow you to analyze a file on your computer, and at @10:20, you’ll click analyze next to the process button, and then click measure.
Advanced Bioinformatics Activity 3: Primer Design
30-60 minutes

Prepare
- Make sure you still have the ApE software from the first bioinformatics exercise installed on your computer.
Watch the video & complete the exercise.e - Go to this YouTube link: Amino Labs Bioinformatics: Primer Design Tutorial and watch the video to follow along to complete the exercise.
Storage, Disposal, Clean Up
After everyone sees their results, all experiment materials, including gels, tubes, and pipette tips, should be in the discard containers. Disposing of experiment materials is an integral part of the experiment. Always wear gloves for cleanup!
- Reusable materials: If you have DNA, primers, loading dye, ladders, and ultrapure water, they can last up to 6 months when stored in a refrigerator. Make sure it has not been contaminated before you store it. For example, if you reused a pipette tip when pipetting from one of these tubes, it may now be contaminated. To store and keep these, place them in a ziploc bag inside a sealed plastic container in a refrigerator away from food items. If you do not wish to keep it, addit to an inactivation bag. Make sure the lids are separate from the tubes so that the inactivating liquid can get inside. Follow the inactivation instructions below.
If you are out of inactivation bags, use a sturdy ziploc-type bag or a disposable container with a lid. Always wear gloves when handling experimental materials and cleaners! - Unused ingredients: If you did not use all the mastermix tubes, store these for later use. Store them in their Ziploc bag within a sealed container in the refrigerator for up to 6 months.
- Inactivation: Transfer the tubes, gels, samples, and pipette tips in the discard containers to an inactivation bag. Paper packaging, plastic bags, and gloves go in the regular garbage or recycling. Make sure all the tubes have their lids off once in the inactivation bag, and add a solution of 1 part bleach to 4 to 6 parts water to the inactivation bag. Close the bag and let it sit for 24 to 48 hours before discarding the liquid in the toilet and the solids & bags in the garbage. Step-by-step instructions are on the inactivation bag and in an inactivation video on our YouTube channel: youtube.com/AminoLabs.
- Clean the discard containers. Spray some chlorinated bleach cleaner in the discard container(s) once emptied. Let it sit for an hour before wiping down. You can wait to wipe it down until you empty your inactivation bags the next day.
- Clean your workspaces: Use a chlorinated spray cleaner, wipes, or a solution of 1 part chlorinated bleach to 9 parts water to wipe down your work area. If you have them, you can wipe down the DNA Playgrounds with this solution and follow it with an eyeglass or window cleaner to remove the inevitable streaking from the bleach cleaner. Never use rubbing alcohol (isopropyl alcohol) on the DNA Playgrounds.
Troubleshooting
Here’s a short troubleshooting guide for some common problems that can occur during PCR:
No amplification
If you’re not seeing any amplification of your target DNA, there may be a problem with your primers. Check to make sure they are designed correctly and annealing to the target sequence properly. You may also need to optimize the PCR conditions, such as the annealing temperature or the extension time.
Weak amplification
If you’re seeing weak amplification of your target DNA, you may need to optimize the PCR conditions. Increasing the annealing temperature or the extension time may help improve amplification. You may also need to adjust the primer concentrations or the amount of template DNA in your reaction.
Non-specific amplification
If you’re seeing amplification of non-target DNA, there may be a problem with your primers or PCR conditions. Check to make sure your primers are specific to your target sequence and not annealing to other regions of DNA. You may also need to optimize the annealing temperature or the extension time to reduce non-specific amplification.
Contamination
Contamination can be a common problem in PCR, especially when working with small amounts of DNA. Be sure to use sterile techniques when setting up your reaction and avoid cross-contamination between samples. Use separate pipettes, tips, and workspaces for each sample, and sterilize your equipment and workspace regularly.
PCR inhibition
PCR inhibition can occur when there are inhibitors present in your sample, such as proteins or salts. To avoid this, purify your DNA template before PCR and use clean, high-quality reagents for your reaction. You may also need to adjust the reaction conditions to optimize amplification.
Remember, troubleshooting PCR can be a complex process, and there are many factors that can affect the success of your reaction. By carefully optimizing your PCR conditions and troubleshooting any problems that arise, you can obtain high-quality results for your research.
If you need help, please contact us: [email protected]
For more manuals by Amino Labs, visit ManualsLibraryy
Amino Labs PCR-IT KIT Classroom- FAQs
Q1. What is the use of the PCR teaching kit?
It is used to amplify a specific DNA or RNA fragment through three steps—denaturation, annealing, and extension—helping students learn molecular biology techniques.
Q2. Can Amino Labs kits be used at home?
Yes. The kits are designed for classrooms and homes, safe for ages 10 and up, and do not require a special lab setup.
Q3. Are Amino Labs PCR kits safe?
Yes. They only use non-pathogenic components that fall under Biosafety Level 1 (BSL1), making them safe for educational use.
Q4. What are the basic steps of PCR?
PCR involves three main steps: denaturation (DNA separation), annealing (primer binding), and extension (DNA synthesis).
Q5. What materials are needed for PCR?
The essential components are template DNA, primers, DNA polymerase, nucleotides (dNTPs), buffer, and magnesium ions.
Q6. How does PCR work in simple terms?
PCR makes many copies of a specific piece of DNA, so even tiny amounts can be detected and studied.
Q7. What is the full form of PCR?
PCR stands for Polymerase Chain Reaction.
Q8. What is a PCR machine?
A PCR machine, or thermal cycler, heats and cools DNA samples in cycles to enable DNA amplification.
Q9. What are some applications of PCR?
PCR is used in diagnostics, genetic research, forensic testing, cloning, sequencing, and studying gene expression.


 Mix your sample
Mix your sample
 Run your PCR samples.
Run your PCR samples.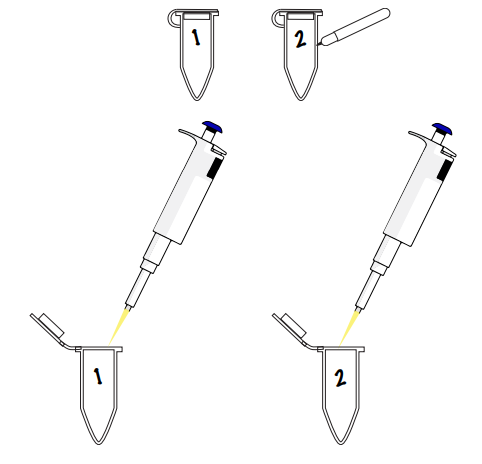
 Load your PCR products and ladder into a gel..
Load your PCR products and ladder into a gel..