Dartwood Upper Arm Electronic Blood Pressure Monitor Instructions
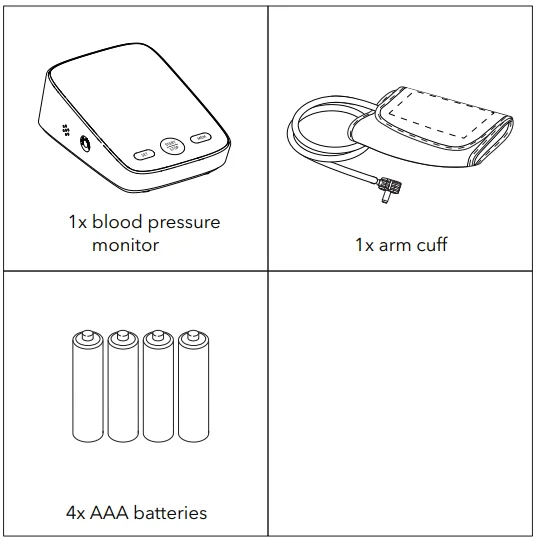
In the Box
Intended Use
- Intended for blood pressure and pulse rate measurement in adults.
- Identifies irregular heartbeats, provides a warning signal.
- For home use only.
Safety Information
Important Safety Notices
To ensure proper usage of the product, it is important to follow the basic safety measures and precautions outlined below:
- Before using the unit, it is essential to thoroughly read all the information provided in the instruction manual and any additional literature included in the box.
- Please consult your physician for specific information regarding your blood pressure. Engaging in self-diagnosis and treatment based solely on measurement results can be hazardous. Always adhere to the instructions provided by your healthcare provider.
- Please ensure that you operate the unit solely for its intended purpose and avoid using it for any other applications. The unit is designed specifically for measuring blood pressure and pulse rate in adult individuals, and it is not recommended for use with newborns or infants, either at home or in a medical center.
- To prevent incorrect operation of the unit, avoid using mobile phones or other devices that emit electromagnetic fields in proximity to the unit.
- To ensure accurate readings, please refrain from using the unit in areas with high levels of radiation.
- Do not disassemble or attempt to repair the unit or its components. Do not use the equipment in areas where flammable gases (such as anesthetic gas, oxygen, or hydrogen) or flammable liquids (such as alcohol) are present.
- It is important to note that excessive frequency in measurements can potentially interfere with the blood flow, leading to possible injury to the patient.
- It is advised not to place the cuff over a wound, as doing so may worsen the injury.
- Please be mindful of the potentially harmful effects caused by continuous cuff pressure due to tangling of the connection tubing, which can interfere with blood flow and result in injury to the patient.
- The adapter must meet the following conditions as per the requirements: the output voltage should be DC 5V, the current should be 1000mA, and it must comply with IEC 6060 -1 and IEC 60601-1-11 standards. Additionally, it must provide two MOPP (Means of Patient Protection) insulation between the AC input and DC output.
- Please ensure that the battery is installed correctly.
- When the battery power is depleted, please replace it with four new batteries.
- If the battery has not been used for over three months, it is advisable to remove the battery. Prolonged inactivity may lead to issues such as leakage, overheating, rupture, and potential damage to the blood pressure monitor body.
Warnings and Safety Precautions
- When applying the cuff and exerting pressure on any limb that has vascular access, therapy, or an arteriovenous (A-V) shunt, exercise caution. Temporary interference with blood flow in such cases can potentially lead to injury to the patient.
- When applying the cuff and exerting pressure on the arm, specifically on the side of the brachial artery, please take care and follow proper procedures.
- The pressurization of the cuff may temporarily interfere with the functioning of other medical monitoring equipment simultaneously used on the same limb.
- It is important to check, such as through observation of the relevant limb, that the operation of the automated blood pressure monitor does not lead to prolonged impairment of the patient’s blood circulation.
- If the user’s arm becomes uncomfortable under pressure from an over-inflated cuff, please loosen the cuff or remove the batteries immediately.
- Please avoid touching the patient and the battery output simultaneously during the measurement process, as this may interfere with the reading.
- Do not use lure connectors. If lure lock connectors are utilized in the tubing construction, there is a risk of accidental connection to vascular fluid systems, potentially leading to the introduction of air into a blood vessel.
- Portable RF communications equipment, including peripherals such as antenna cables and external antennas, must be kept at a minimum distance of 30 cm (12 inches) from any part of the Blood Pressure Monitor, including cables specified by the manufacturer. Failure to do so may result in a degradation of the performance of the equipment.
- Ensure that the Blood Pressure Monitor is kept out of reach of children and animals.
- There is a risk of accidental ingestion of small disassemblable parts, such as batteries.
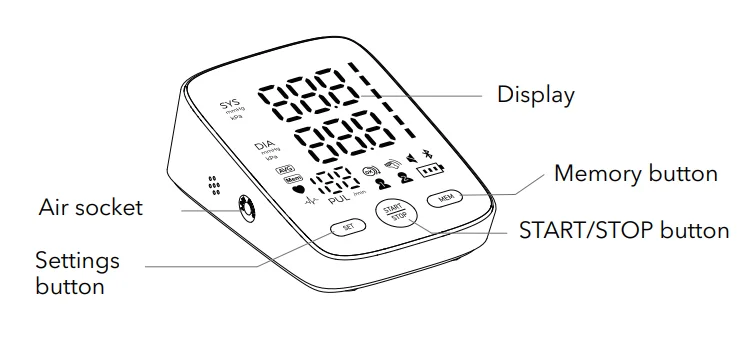
Introduction to Components
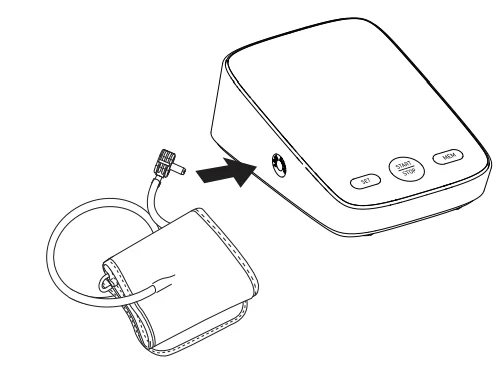
Main unit (front)
Arm cuff
The cuff fits upper arm sizes between 8.7 – 16.5 inches (22cm – 42cm)

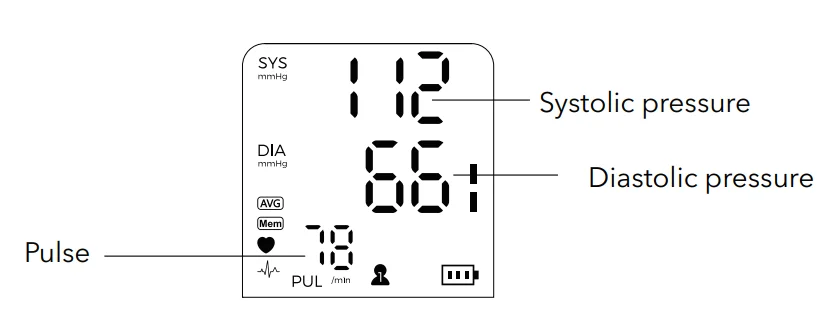
Display

Battery Handling and Usage Guidelines
- Ensure correct alignment of battery polarities when inserting them.
- Use only 4 AAA alkaline or manganese batteries with this monitor. Do not use any other types of batteries.
- Do not mix new and used batteries.
- Do not mix different brands of batteries.
- If the blood pressure monitor is not to be used for an extended period, please remove the batteries.
- Do not use batteries past their expiration date.
- Regularly inspect the batteries to ensure they are in good working condition.
Before Use
Install the batteries
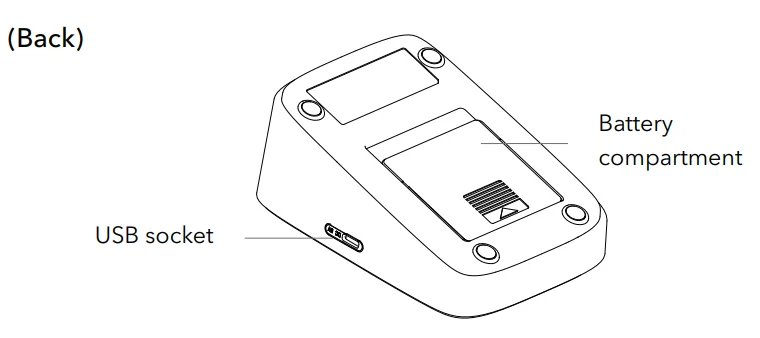
- To access the battery compartment, remove the battery cover.
- Install four AAA-size batteries, ensuring that the + (positive) and – (negative) polarities align with the corresponding markings.
- Securely replace the battery cover.

Note: If the low battery symbol appears on the display, promptly replace the batteries.

Applying the arm cuff
- Ensure the air plug is properly inserted into the main unit.

- Before applying the cuff, remove any clothing from your upper arm, allowing the cuff to make direct contact with the skin.

- Sit in a chair with your feet flat on the floor. Position your arm on a table; the cuff should be level with your heart.

- Thread the hose through the metal loop and ensure that the hose extends outward.

- Slide your arm through the loop and pull the cuff up to the position of your upper arm

- The hose should run down the inside of your arm along the main arteries. Position the bottom of the cuff approximately 0.8-1.2 inches (2~3cm) above your elbow.

- Wrap the cuff tightly around your upper arm using the Velcro strip. Ensure that there is a space of 1-2 fingers between your arm and the cuff.

- Relax your arm and keep your palm facing upward. Let your fingers naturally curve. Turn on the unit and start the measurement.
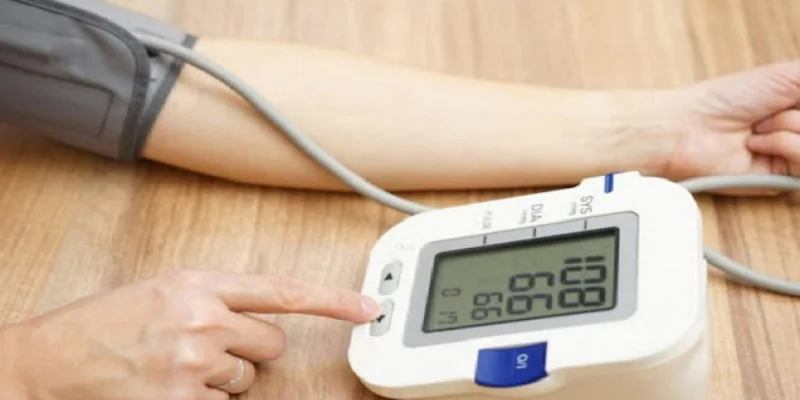
Taking the Measurement
- Press ‘START/STOP’ to begin. All symbols will display, and the cuff will inflate automatically for measurement.

- After measurement, blood pressure and pulse rate will be displayed and stored in memory. Cuff deflates automatically.
 *
*  icon signals irregular heartbeat during measurement.
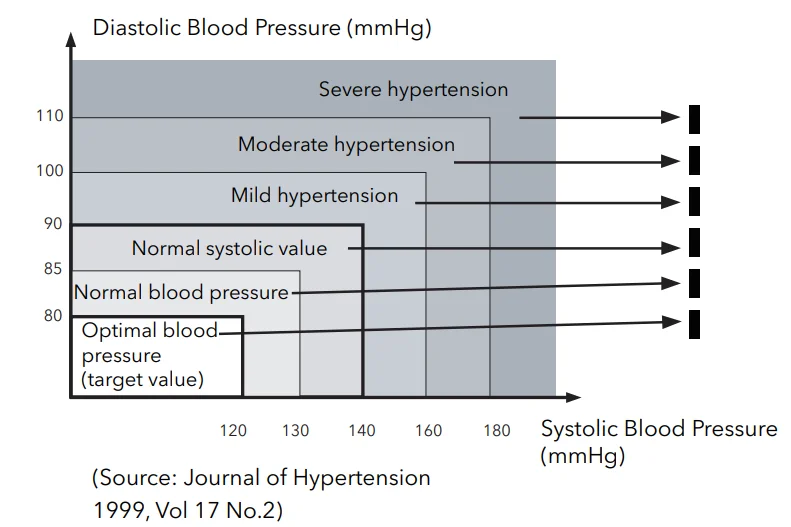
icon signals irregular heartbeat during measurement.Blood Pressure Indicator
After each measurement, the LED display shows your position on the WHO Blood Pressure Indicator bar. See below.


- To power off, press “START/STOP”. The device will also automatically turn off after three minutes of inactivity.
Switch User
To switch users, power off the unit, then press the setting button to toggle between “1” and “2”. Confirm by pressing “START/STOP”.

Memory Function Overview
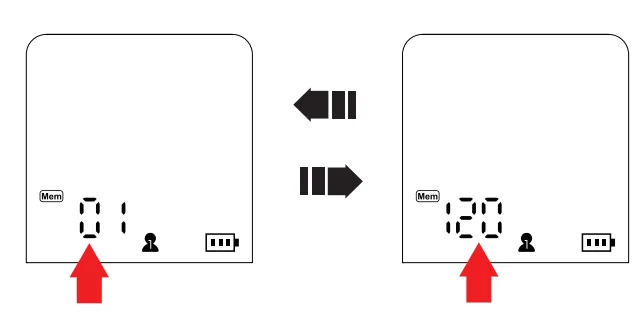
- To view the average of the last 3 measurements, power off and press the memory button. The display will show the average.

- Press the memory button again to access records.
- The display shows “01” for the latest record.
- Use the memory button to navigate through records.
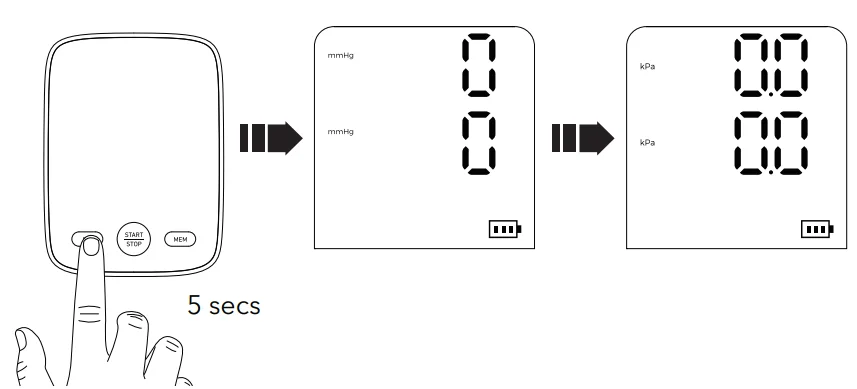
Change the Measurement Unit
- Change unit (kPa or mmHg) by holding the setting button for 5s when off.
- Display flashes for unit setting.
- Press the memory button to choose the unit.
- Confirm with “START/STOP”.
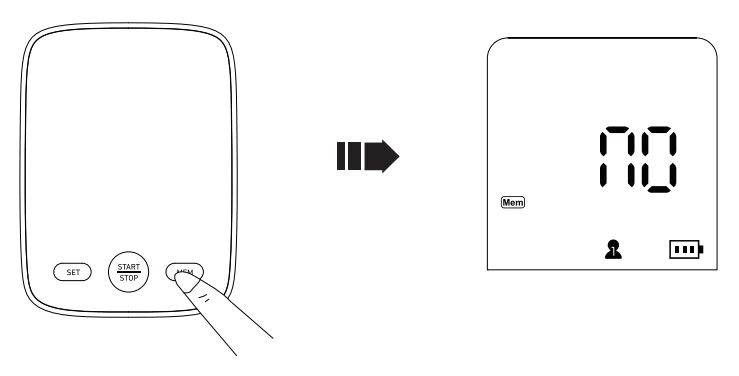
Clearing the Memory
Note: Ensure you want to delete all records.
Switch user, then hold the memory button for 10 seconds. “NO” displays when all records are deleted.
Cleaning/Disinfecting and Maintenance
For optimal performance and protection:
- Clean the monitor and cuff after use.
- Avoid abrasive cleaners.
- Never submerge in water.
- Wipe with a soft, damp cloth.
- Disinfect the cuff with 75% alcohol cotton.
- Follow manual instructions; use authorized parts.
Storage and Maintenance
- Store unit in a dry place; do not fold cuff tightly.
- Shield from extreme temperatures, humidity, and sunlight.
- Avoid dropping; protect from impacts.
- If unused for 3 months, replace batteries.
- Follow instructions, use authorized parts.
Tips
- For consistent readings, use the same arm.
- If significant difference, consult a physician for future measurements.
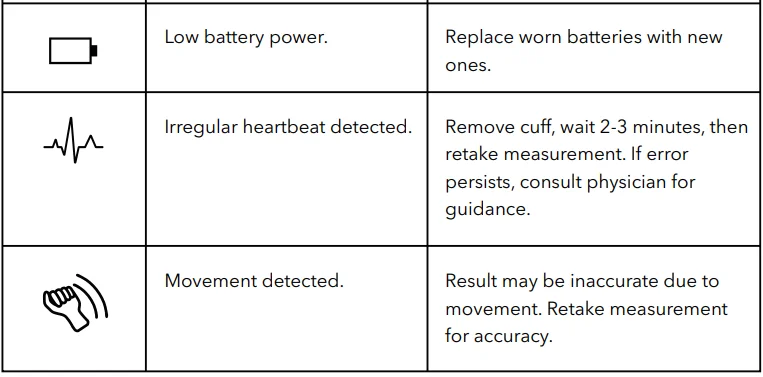
Error Codes

IEC 60601-1-2:2014/AMD1:2020 Medical electrical equipment (ME EQUIPMENT) and medical electrical systems (ME SYSTEMS) identification, marking, and documents for Class B product.
Instructions for Use
This ME EQUIPMENT / ME SYSTEM device is designed to be appropriate and safe for use in home healthcare environments
WARNING: Keep the device a safe distance from active high-frequency (HF) surgical equipment and the radio frequency (RF) shielded room of an ME system for magnetic resonance imaging, as the intensity of electromagnetic disturbances is high.
WARNING: Avoid using this equipment adjacent to or stacked with other equipment, as it may lead to improper operation. If such use is necessary, carefully monitor both this equipment and the other equipment to ensure they are operating normally.
WARNING: Using accessories, transducers, and cables other than those specified or provided by the manufacturer of this equipment may lead to increased electromagnetic emissions or reduced electromagnetic immunity, potentially causing improper operation. Please adhere to the manufacturer’s recommendations to ensure proper functionality and safety.
Warning: Portable RF communications equipment, including peripherals such as antenna cables and external antennas, should not be used closer than 12 inches to any part of the Upper Arm Electronic Blood Pressure Monitor (FC-BP121), including cables specified by the manufacturer. Failing to adhere to this distance may lead to a degradation of the performance of this equipment.
If applicable, the Responsible Organization must provide a comprehensive list of all cables and their maximum lengths, transducers, and other replaceable ACCESSORIES that have the potential to affect the compliance of the ME EQUIPMENT or ME SYSTEM with the requirements of Clause 7 (EMISSIONS) and Clause 8 (IMMUNITY). These ACCESSORIES can be specified either generically (e.g., shielded cable, load impedance) or specifically (e.g., by MANUFACTURER and EQUIPMENT OR TYPE REFERENCE). It is crucial to ensure that the replacement of these components does not compromise the compliance of the medical equipment or system with the relevant emission and immunity standards.
If any: the performance of the ME EQUIPMENT or ME SYSTEM that was determined to be ESSENTIAL PERFORMANCE and a description of what the OPERATOR can expect if the ESSENTIAL PERFORMANCE is lost or degraded due to EM DISTURBANCES (the defined term “ESSENTIAL PERFORMANCE” need not be used).
Technical Description
- All necessary instructions for maintaining BASIC SAFETY and ESSENTIAL PERFORMANCE about electromagnetic disturbances for the expected service life.
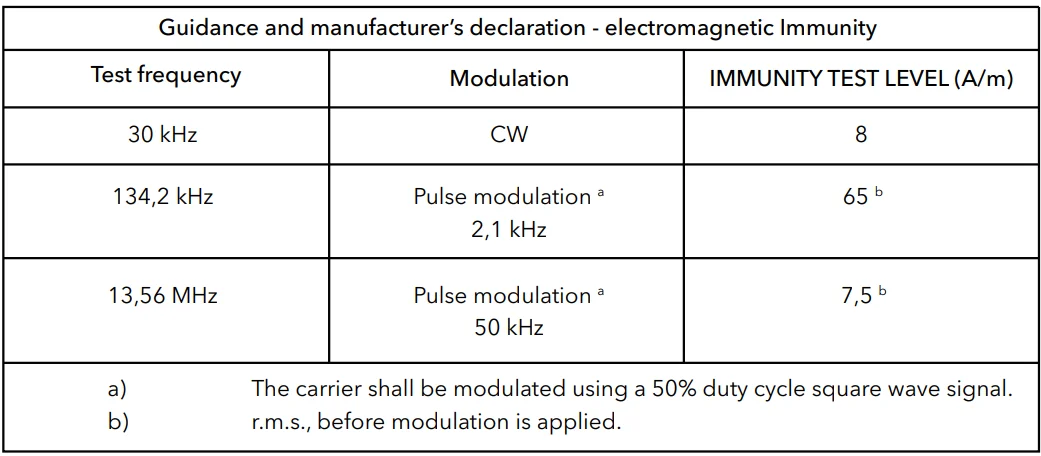
- Guidance and manufacturer’s declaration – electromagnetic emissions and immunity.
Table 1
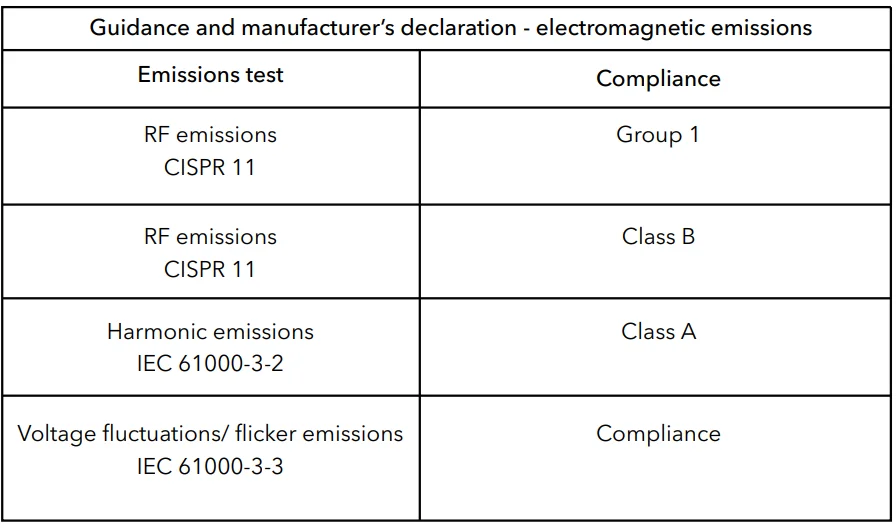
Table 2
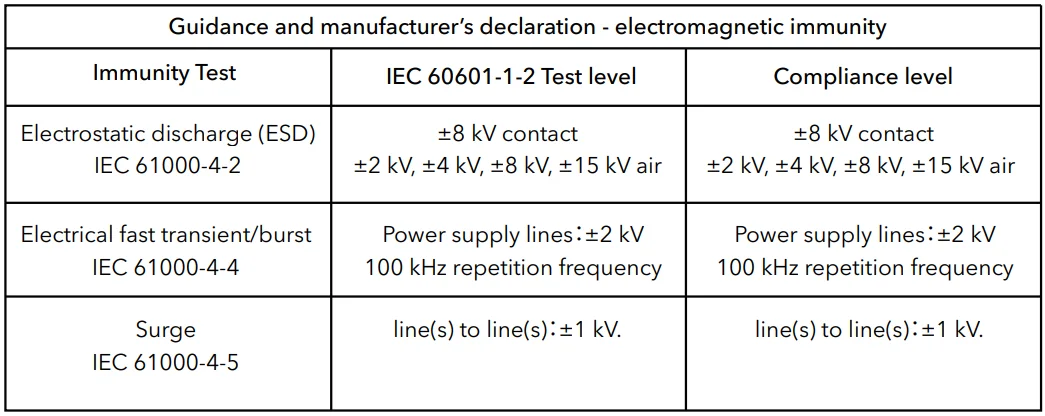
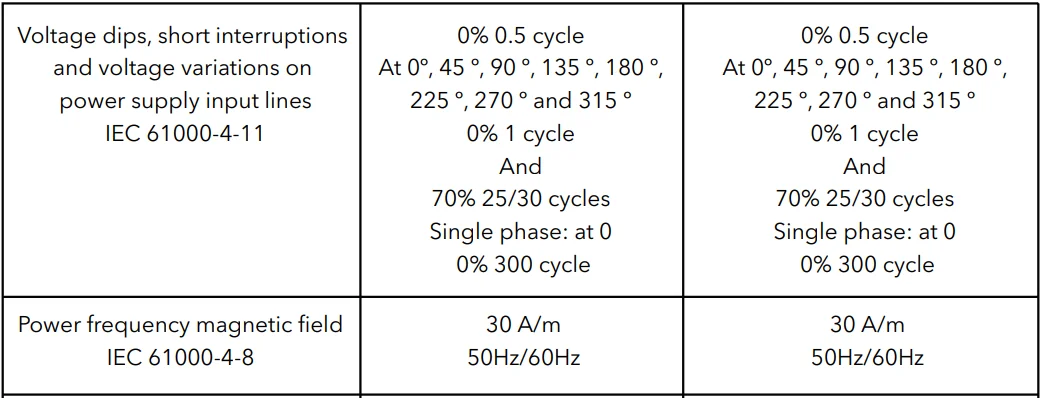
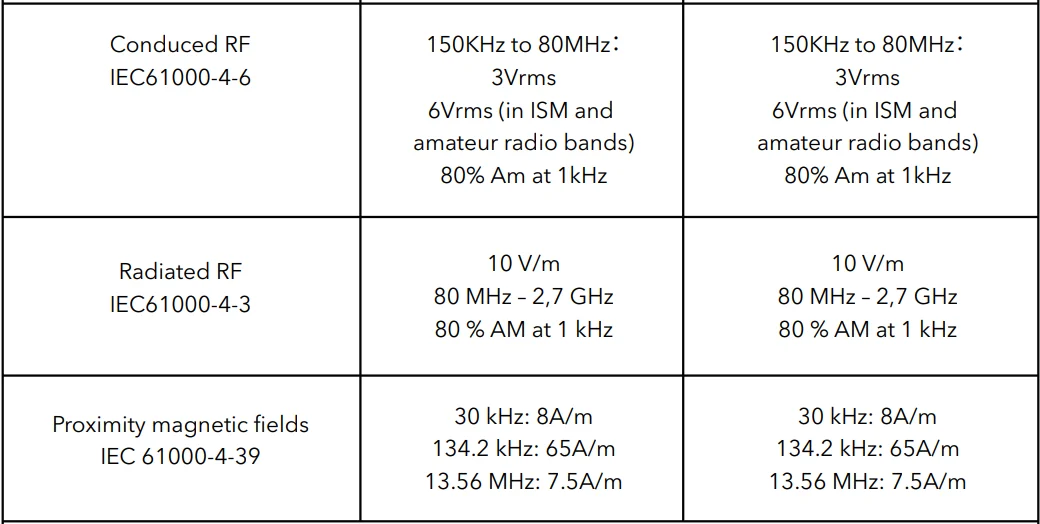
NOTE UT is the a.c. Mains voltage before application of the test level.
Table 3
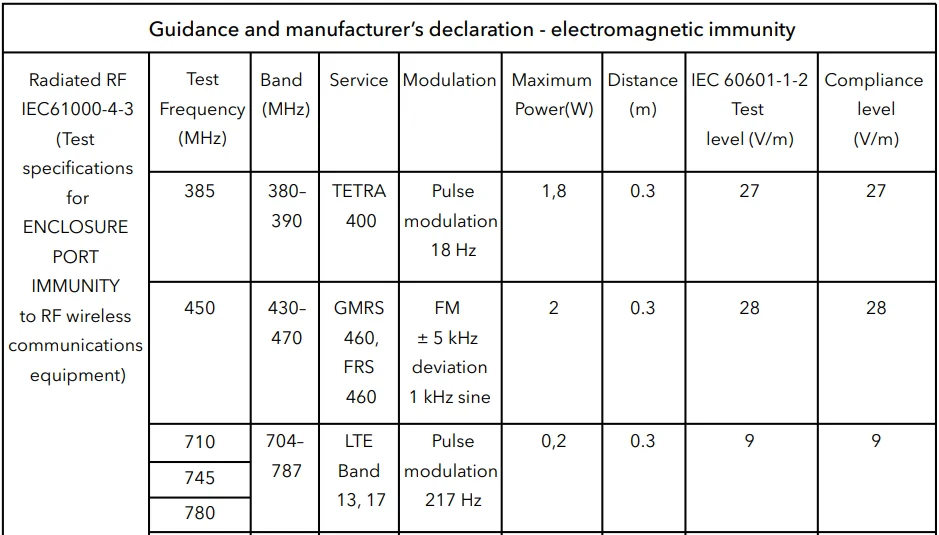
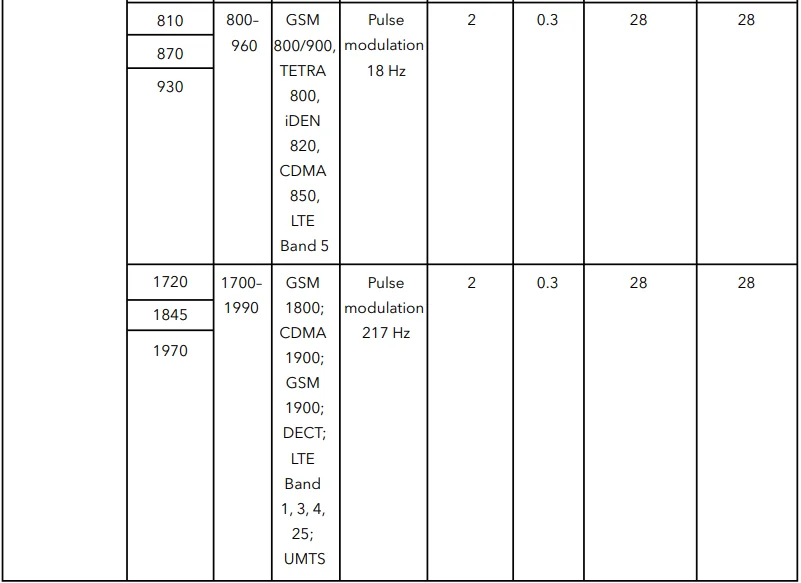
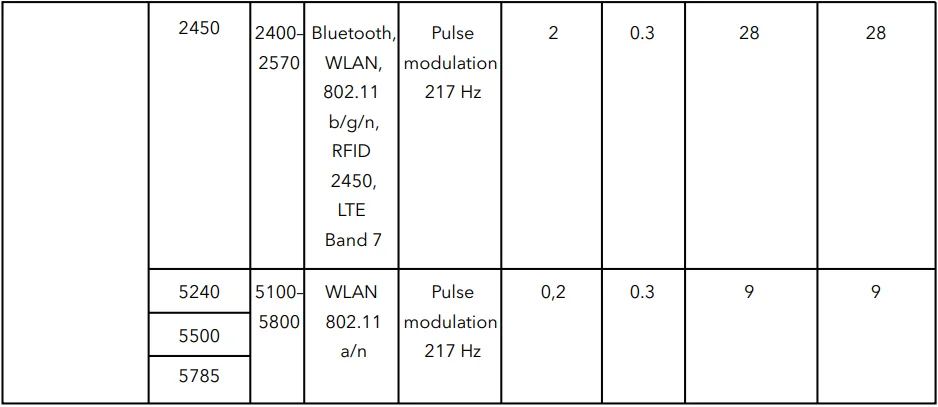
Table 4
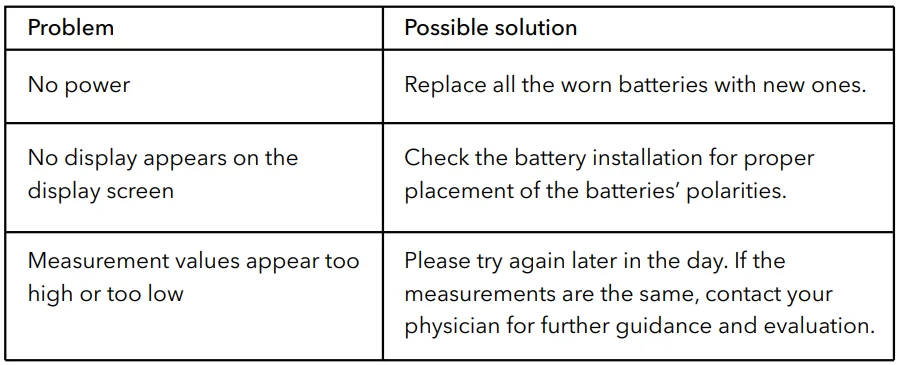
Troubleshooting
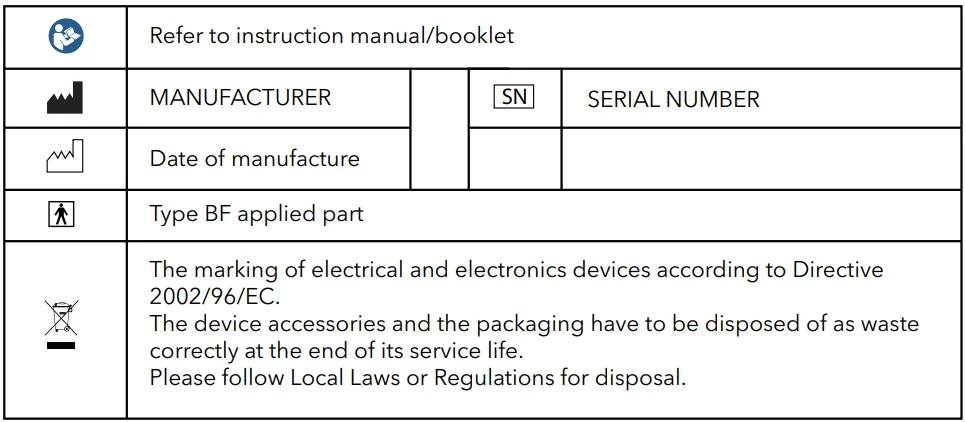
Symbols and Explanation
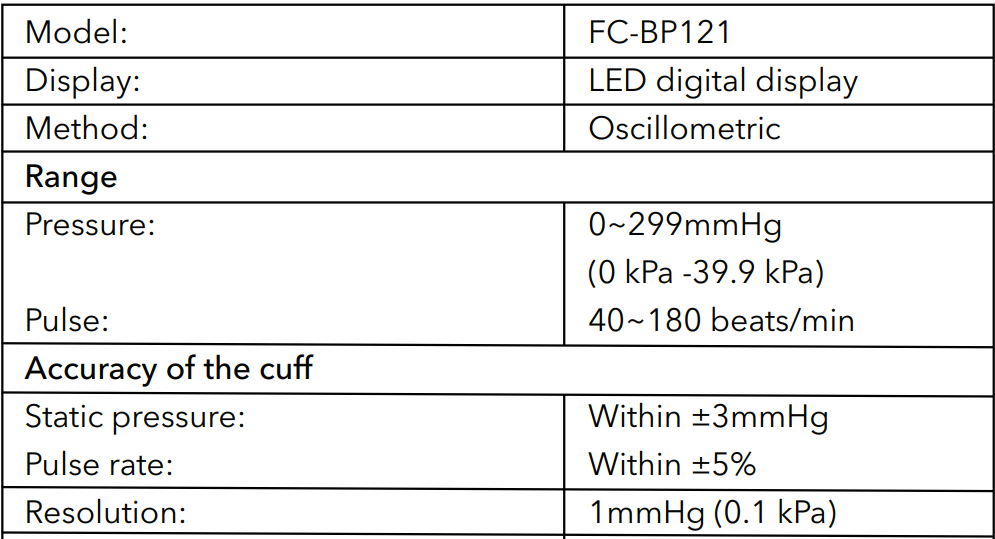
Specifications
U.S. Agent:
CTI U.S. Inc.
Suite 230, 1455 Cti U.S. Inc.
Lincoln Parkway, Atlanta, Ga, 30346
Manufacturer:
Shenzhen Finicare Co., Ltd
201, No.50, the 3rd Industrial Park, Houting Community, Shajing Street, Bao’an District, Shenzhen 518104 China
E-mail: [email protected]
Website: https://www.finicare.com/
Get More Support
Scan the QR code below or email us at [email protected]
For more manuals by Dartwood, visit ManualsLibraryy
Dartwood Upper Arm Electronic Blood Pressure Monitor Instructions- FAQs
How do I use the Dartwood upper arm blood pressure monitor?
Sit comfortably, stretch out your arm with palm up, and wrap the cuff around your bare upper arm about 1 inch above the elbow. Make sure the tubing runs over the center of your arm. Press the start button to measure.
How should I measure blood pressure for the first time?
Charge the device if needed, place the cuff correctly on your arm, remain relaxed, and take 2–3 readings at 1–2 minute intervals. Record the readings for accuracy.
What is an XXL blood pressure cuff?
An XXL cuff fits larger arm sizes, typically 9–24 inches (22–60 cm) in circumference, ensuring accurate readings for people with bigger arms.
Can a tight or small cuff affect readings?
Yes. A cuff that is too tight may give falsely high readings, while a cuff that is too loose may underestimate blood pressure. Always use the correct size.
When is the best time to check blood pressure?
Measure in the morning before eating or taking medicine and again in the evening. Avoid exercise, caffeine, and smoking 30 minutes prior to measurement.
Why is my first reading often higher than the second?
Factors like stress, white coat syndrome, or a full bladder can raise your first reading. Multiple measurements give a more accurate result.
Which arm should I use to measure blood pressure?
The right arm is generally preferred, especially for women, but consistency is key—measure from the same arm each time.
Can caffeine, water, or anxiety affect readings?
Yes. Coffee, energy drinks, dehydration, or temporary anxiety can temporarily raise blood pressure. Relax and hydrate before measuring.







 *
*