
Honeywell HCiR series CFR Part 11 FDA Electronic Records-Signatures

Using Honeywell HCiR series to create 21 CFR Part 11 FDA-compliant Application: Electronic Records & Signatures
Honeywell Modular system solution “Controller + HMI” is an optimized and outstanding solution for Pharmaceutical and Life Science manufacturers requiring a cost-effective system that is fully compliant with 21 CFR Part 11 FDA. Honeywell HCiR series can reach this application programming Environment, HCiR allows the system to be validated to the 21 CFR Part 11 regulations by featuring functions and features that enable.
USER MANAGEMENT FUNCTIONALITIES:
- Password-protected individual, unique user account.
- Password complexity
- Configure a character count for a password with Flexibility
- Configurable trials to modify passwords for user accounts
- Password validity
- Multiple password levels for each User Authority
AUDIT TRAIL (TRACKING):
- Non-editable Audit Trail Data Format
- The time-stamp of the modification of the parameter value and the user making the modification
- The Audit Trail records the following details:
- User creation
- User Login/Logout
- Configurable block by Administrator
- The old value and the new value of the parameter change
- The time-stamp of each event
SYSTEM DATA AND DATA BACKUP:
HCiR offers the basic connectivity for data exchange with Honeywell solutions, Experion, ControlEdge PLC, RTU, HC900, and MasterLogic PLC by following:
- Communication: Modbus
- Data file Transfer (FTP)
- USB, SD card
ELECTRONIC DATA RECORD AND STORAGE:
Review of the reports on the HMI Screen for Production, Alarms, and event (trail). And also, these data are available to save into an SD card, and the user can copy/move the data to external memory (USB).
VIEW OF THE ALARM/EVENT DATA:
Detected alarm/event data is stored in internal memory. Capture and Printing: The captured screen is available to print out online through a printer to support the PCL format.
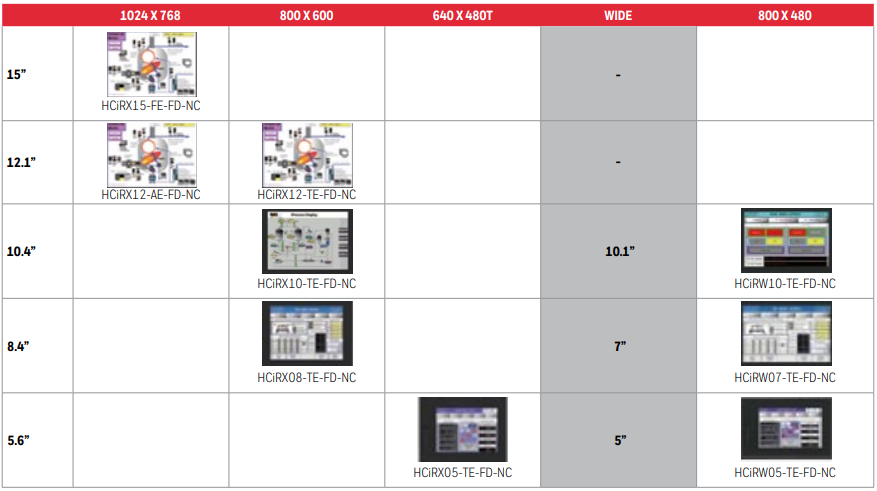
PRODUCT LINE

COMPLIANT
Solutions to facilitate validation and reduce compliance costs with system self-documentation, electronic change management of control configuration, 21 CFR part 11 compliance and enforcement, and verification of operating instructions.
FAST
A unique approach to project execution with LEAPTM project execution and technologies such as our Universal I/O removes risks and accelerates schedules.
VIRTUAL READY
A Virtual Engineering Platform allows for virtualization and simulation of all DCS components for cost-effective change implementation in a validated environment.
EXPERIENCED
More than a decade working with the world’s leading pharma businesses, with specialists who bring a lifetime’s experience in pharma.
SCALABLE
Delivering the most advanced technologies to the smallest and largest operations, with flexible solutions.
SECURE
Industry-leading cyber security, access control, and protection against message flooding and denial of service to ensure system availability.
For more information, process.honeywell.com
For More Manuals By Honeywell, Visit ManualsLibraryy.
Honeywell HCiR series CFR Part 11 FDA Electronic Records-Signatures-FAQs
What does CFR Part 11 cover?
21 CFR Part 11 sets FDA regulations for the use of electronic records and electronic signatures, ensuring their authenticity, integrity, and security.
What makes an electronic signature Part 11 compliant?
It must be secure, trustworthy, and reliable, with proper system validation, audit trails, and access controls.
What are the benefits of Part 11 compliance?
It improves data integrity, security, and efficiency by allowing businesses to digitize record-keeping and approval processes.
What challenges do organizations face with Part 11 compliance?
Common gaps include weak access controls, incomplete audit trails, system validation issues, and improper user management.
What types of electronic signatures exist?
The three main types are Simple Electronic Signatures (SES), Advanced Electronic Signatures (AES), and Qualified Electronic Signatures (QES).
What are the four key requirements for a valid e-signature?
Intent to sign, consent to use e-signatures, proper authentication, and secure record retention.
What documents cannot be signed electronically?
Generally excluded are wills, property deeds, marriage, birth, and death certificates, or any documents legally requiring wet signatures.
What documents can e-signatures be used for?
They are valid for contracts, authorizations, service agreements, and most business or compliance records

